Limitations of the Rutherford Model of the Atom 🧪
Explore the key drawbacks of Rutherford's atomic model and understand why it couldn't explain atomic stability and spectral lines based on electromagnetic theory.
Rebiaz Studio
36 views • Apr 21, 2025
About this video
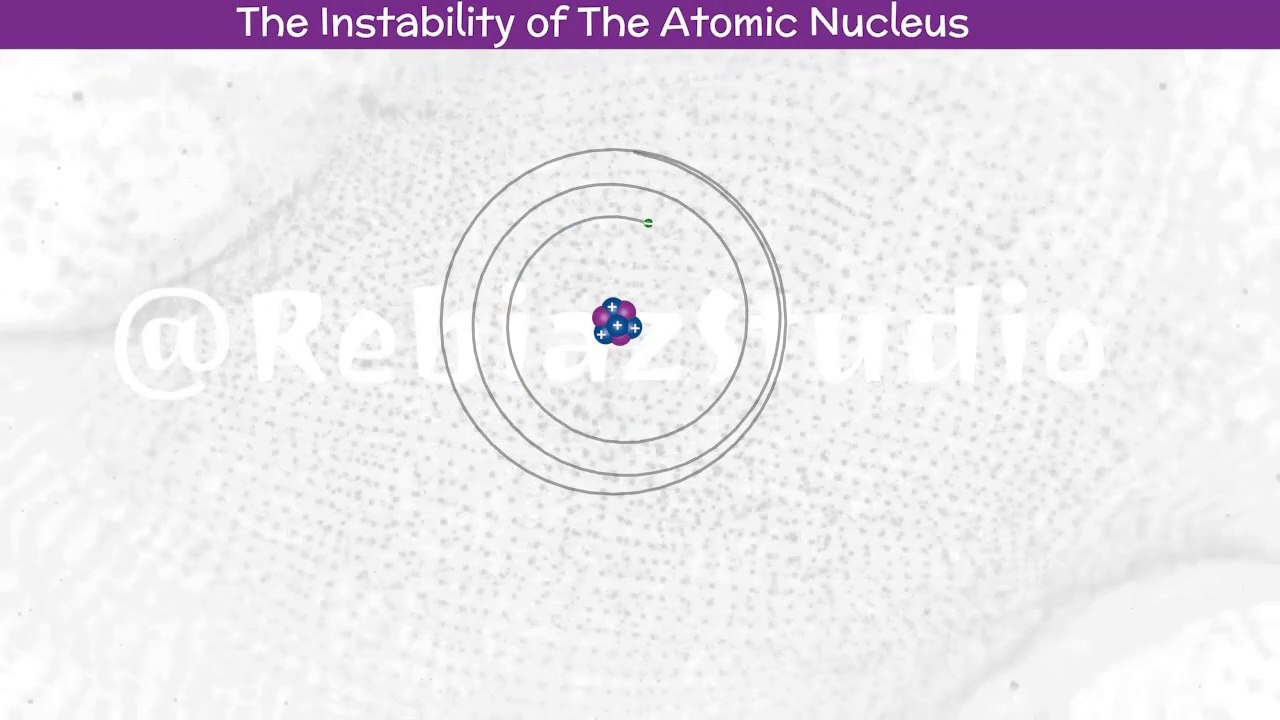
According to Maxwell theory of electromagnetic radiation, a charged body moving under the influence of attractive forces loses energy continuously in the form of electromagnetic radiation. Thus, the electron is a charged body and it should emit radiations while revolving around the nucleus. As a result of this, the electron should lose energy at every turn and move closer and closer to the nucleus following a spiral path. Consequently, the orbit will become smaller and smaller and finally the electron would fall into the nucleus. In other words, the atom should collapse.<br /><br />Watch the video related to *STRUCTURE OF ATOMS* :<br />https://dailymotion.com/playlist/x8m8p2<br /><br />By following this channel it is very useful for the development of this channel. The Dailymotion application is easy to install on any smartphone, and does not take up much memory space.<br />
Video Information
Views
36
Duration
2:58
Published
Apr 21, 2025
Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.
Trending Now