How Aromaticity Leads to Permanent Dipole Moments Explained in 1 Minute 🧪
Discover how aromatic compounds can generate a permanent dipole moment through the principles of Huckel’s Rule. Perfect for quick chemistry insights!

One Chemistry
3.3K views • Feb 15, 2022
About this video
For feedback and business queries, please email us at suviganu@gmail.com
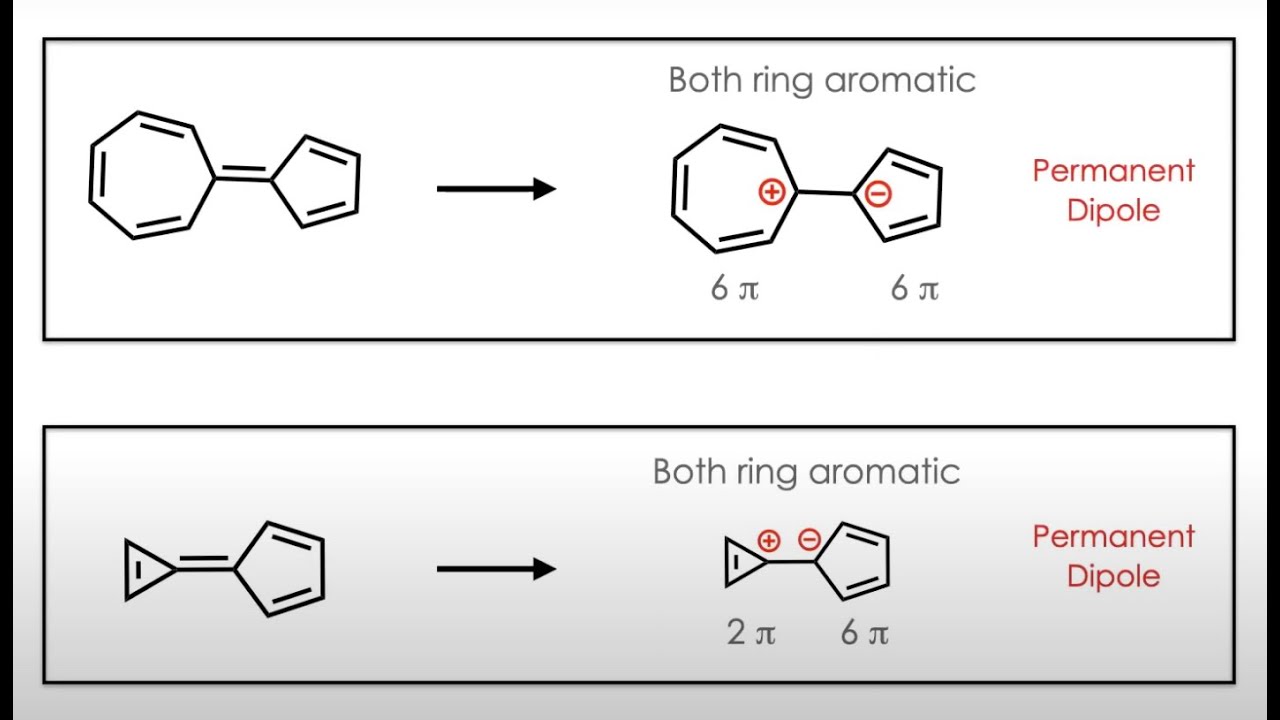
This video is about how the aromaticity can create the permanent dipole moment. Since the Aromaticity can stabilise the charges and can create the permanent stability which in turn create the permanent dipole moment. If the anion and cation can stabilised by aromaticity in the same system, it can create the dipole moment.
Aromaticity and dipole moment are two fundamental properties of molecules that are closely intertwined. Aromaticity, a special type of delocalization of electrons in a cyclic molecule, confers unique properties to the molecule. Dipole moment, a measure of the polarity of a molecule, arises from the uneven distribution of electron density.
Aromaticity and Electron Delocalization
Aromatic molecules are characterized by a cyclic arrangement of atoms with alternating single and double bonds, and they obey Hückel's rule (4n+2 π electrons). This delocalization of electrons creates a cloud of electron density above and below the plane of the molecule, resulting in a highly stable system.
Influence of Aromaticity on Dipole Moment
Symmetric Aromatics:
Zero Dipole Moment: Aromatic molecules with high symmetry, such as benzene, have a zero dipole moment. This is because the electron density is evenly distributed around the ring, canceling out any individual bond dipoles.
Example: Benzene
Unsymmetric Aromatics:
Non-Zero Dipole Moment: Aromatic molecules with lower symmetry, such as toluene or naphthalene, have a non-zero dipole moment. The electron-donating or electron-withdrawing nature of substituents can disrupt the symmetrical distribution of electron density, creating a dipole.
Aromatic Heterocycles:
Dipole Moment: Aromatic heterocycles, containing atoms other than carbon in the ring (e.g., nitrogen, oxygen), often exhibit dipole moments due to the electronegativity differences between the ring atoms.
Factors Affecting Dipole Moment in Aromatics
Substituent Effects: Electron-donating or electron-withdrawing substituents on the aromatic ring can significantly influence the dipole moment.
Ring Size and Fusion: The size and fusion of aromatic rings can also affect the dipole moment.
Conformational Effects: In some cases, the conformation of the molecule can influence the dipole moment.
This video is part of one minute chemistry series. Enjoy Quick learning. .
For business queries, send message to suviganu@gmail.com with your phone number . We will call you back.
Office Address
Dr. B. Ganesh Kumar,
1255, Third street, Asari Colony,
Satchiyapuram, Sivakasi- 626124
Tamil Nadu, India.
#JEE #NEET #NET #GATE #One #OneChemistry #JAM #Chemistry
Subscribe to my YouTube channel: https://www.youtube.com/c/OneChemistry?sub_confirmation=1
Content Creation: Prof. GKR
Please feel free to give comments and feedback.
__________________________________________________________________________
The creator of this channel Prof. GKR, his Ph.D in Chemistry from Hyderabad Central University and cleared CSIR-JRF and NET. He is currently Associate Professor in the P.S.R Arts and Science College, Sivakasi, Tamil Nadu.
## Know One Chemistry ##
Youtube : https://www.youtube.com/c/OneChemistry
Facebook: https://www.facebook.com/wingsGKR/
Instagram: https://www.instagram.com/onechemistrygkr/
Twitter: https://twitter.com/OneChemistry
Website: https://sites.google.com/view/suviganu/home
Email: suviganu@gmail.com
___________________________________________________________________________
#chemistry #chemistryconcepts #jeeneetchemistry #organicchemistry #TRB #gate #netexam #chemistrynotes #chemicalreactions #organicchemistrytricks #neet #jee #setexam #setexampreparation #netexampreparation #gateexam #gateexampreparation #netexampreparation #neetexam #neetchemistry #neetchemistrypreparation #gatechemistry #netchemistry #ugcchemistry #jee #jeechemistry #jeechemistryquestions #jamchemistry
The content may help you to clear
UPSC Chemistry
CSIR Chemical Science
UGC Chemical Science
GATE Chemistry
NET Chemical Science
NEET Chemistry
JAM Chemistry
TNPSC Chemistry
TRB CHEMISTRY
JEE Chemistry
This video is about how the aromaticity can create the permanent dipole moment. Since the Aromaticity can stabilise the charges and can create the permanent stability which in turn create the permanent dipole moment. If the anion and cation can stabilised by aromaticity in the same system, it can create the dipole moment.
Aromaticity and dipole moment are two fundamental properties of molecules that are closely intertwined. Aromaticity, a special type of delocalization of electrons in a cyclic molecule, confers unique properties to the molecule. Dipole moment, a measure of the polarity of a molecule, arises from the uneven distribution of electron density.
Aromaticity and Electron Delocalization
Aromatic molecules are characterized by a cyclic arrangement of atoms with alternating single and double bonds, and they obey Hückel's rule (4n+2 π electrons). This delocalization of electrons creates a cloud of electron density above and below the plane of the molecule, resulting in a highly stable system.
Influence of Aromaticity on Dipole Moment
Symmetric Aromatics:
Zero Dipole Moment: Aromatic molecules with high symmetry, such as benzene, have a zero dipole moment. This is because the electron density is evenly distributed around the ring, canceling out any individual bond dipoles.
Example: Benzene
Unsymmetric Aromatics:
Non-Zero Dipole Moment: Aromatic molecules with lower symmetry, such as toluene or naphthalene, have a non-zero dipole moment. The electron-donating or electron-withdrawing nature of substituents can disrupt the symmetrical distribution of electron density, creating a dipole.
Aromatic Heterocycles:
Dipole Moment: Aromatic heterocycles, containing atoms other than carbon in the ring (e.g., nitrogen, oxygen), often exhibit dipole moments due to the electronegativity differences between the ring atoms.
Factors Affecting Dipole Moment in Aromatics
Substituent Effects: Electron-donating or electron-withdrawing substituents on the aromatic ring can significantly influence the dipole moment.
Ring Size and Fusion: The size and fusion of aromatic rings can also affect the dipole moment.
Conformational Effects: In some cases, the conformation of the molecule can influence the dipole moment.
This video is part of one minute chemistry series. Enjoy Quick learning. .
For business queries, send message to suviganu@gmail.com with your phone number . We will call you back.
Office Address
Dr. B. Ganesh Kumar,
1255, Third street, Asari Colony,
Satchiyapuram, Sivakasi- 626124
Tamil Nadu, India.
#JEE #NEET #NET #GATE #One #OneChemistry #JAM #Chemistry
Subscribe to my YouTube channel: https://www.youtube.com/c/OneChemistry?sub_confirmation=1
Content Creation: Prof. GKR
Please feel free to give comments and feedback.
__________________________________________________________________________
The creator of this channel Prof. GKR, his Ph.D in Chemistry from Hyderabad Central University and cleared CSIR-JRF and NET. He is currently Associate Professor in the P.S.R Arts and Science College, Sivakasi, Tamil Nadu.
## Know One Chemistry ##
Youtube : https://www.youtube.com/c/OneChemistry
Facebook: https://www.facebook.com/wingsGKR/
Instagram: https://www.instagram.com/onechemistrygkr/
Twitter: https://twitter.com/OneChemistry
Website: https://sites.google.com/view/suviganu/home
Email: suviganu@gmail.com
___________________________________________________________________________
#chemistry #chemistryconcepts #jeeneetchemistry #organicchemistry #TRB #gate #netexam #chemistrynotes #chemicalreactions #organicchemistrytricks #neet #jee #setexam #setexampreparation #netexampreparation #gateexam #gateexampreparation #netexampreparation #neetexam #neetchemistry #neetchemistrypreparation #gatechemistry #netchemistry #ugcchemistry #jee #jeechemistry #jeechemistryquestions #jamchemistry
The content may help you to clear
UPSC Chemistry
CSIR Chemical Science
UGC Chemical Science
GATE Chemistry
NET Chemical Science
NEET Chemistry
JAM Chemistry
TNPSC Chemistry
TRB CHEMISTRY
JEE Chemistry
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
3.3K
Likes
44
Duration
1:45
Published
Feb 15, 2022
User Reviews
4.4
(3) Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.