Annelation Effect in Aromatic Systems Explained in 1 Minute ⏱️
Discover the Annelation Effect and its impact on aromatic compounds with a quick, easy-to-understand overview of Huckel’s Rule and aromaticity. Perfect for chemistry enthusiasts!

One Chemistry
3.3K views • Feb 12, 2022
About this video
For feedback and business queries, please email us at suviganu@gmail.com
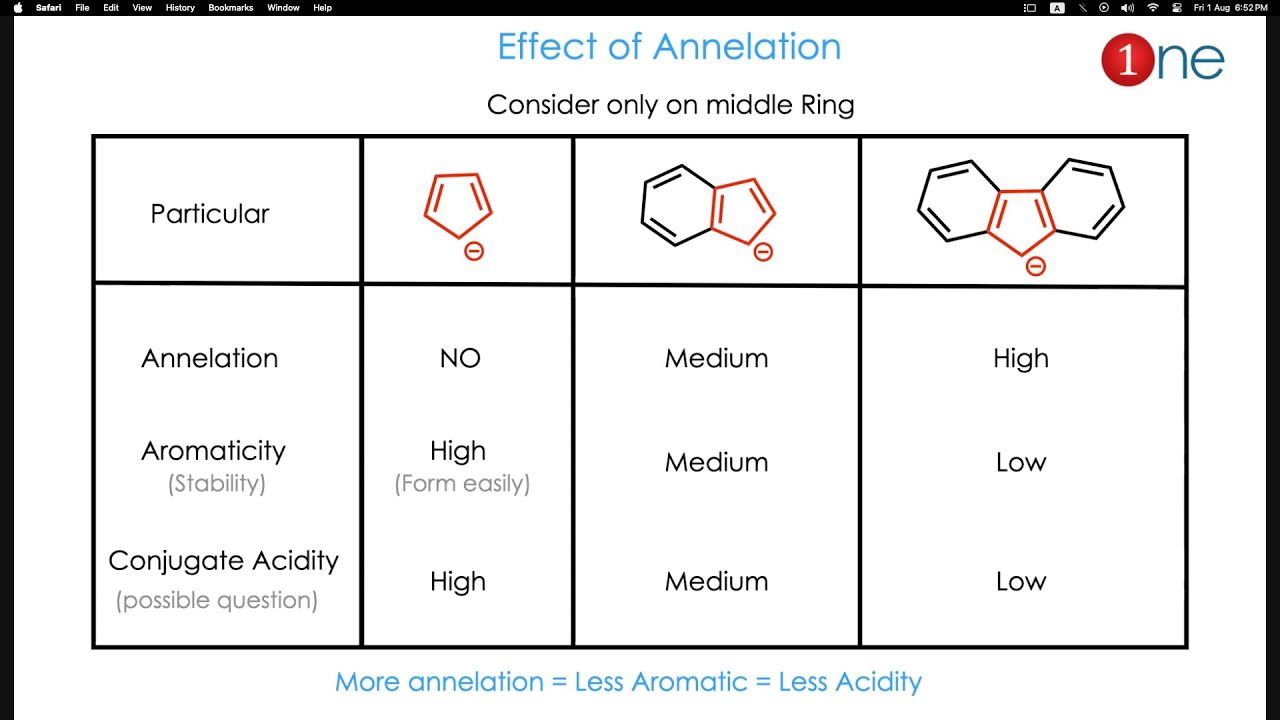
This video is about annelation effect in aromatic system. The annealtion effect is partial loss of aromatic character due to the sharing of electron in polycyclic system.
The annelation effect in chemistry refers to the stabilization or destabilization that occurs when a ring is fused to another molecule. This effect is primarily observed in aromatic compounds and is influenced by factors such as ring size, aromaticity, and the nature of the fused rings.
Aromaticity: The annelation effect is particularly pronounced in aromatic compounds. Fusing a ring to an aromatic system can either enhance or diminish its aromaticity.
Ring Size: The size of the fused ring plays a crucial role. Fusing a five-membered or six-membered ring to an aromatic system generally leads to stabilization, while fusing larger or smaller rings can result in destabilization.
Nature of Fused Rings: The electronic properties of the fused rings also influence the annelation effect. Electron-rich fused rings tend to stabilize the aromatic system, while electron-deficient rings can destabilize it.
Cases of annelation effect:
Naphthalene: When a benzene ring is fused to another benzene ring to form naphthalene, the resulting molecule is more stable than two isolated benzene rings. This is due to the delocalization of electrons over the entire fused system.
Anthracene: Fusing another benzene ring to naphthalene results in anthracene, which is also more stable than its constituent benzene rings. However, the stabilization is not as significant as in naphthalene due to the increased steric hindrance between the fused rings.
Phenanthrene: Fusing a benzene ring to a different position of naphthalene leads to phenanthrene, which is less stable than naphthalene. This is because the fusion introduces some strain into the molecule.
Factors influencing the annelation effect:
Resonance stabilization: The delocalization of electrons through resonance contributes to the stability of fused aromatic systems.
Angle strain: The angles between bonds in fused rings can deviate from the ideal 120° angle, leading to strain and destabilization.
Steric hindrance: Bulky substituents on the fused rings can hinder rotation and increase steric repulsion, destabilizing the molecule.
This video is part of one minute chemistry series. Enjoy Quick learning.
For business queries, send message to suviganu@gmail.com with your phone number . We will call you back.
Office Address
Dr. B. Ganesh Kumar,
1255, Third street, Asari Colony,
Satchiyapuram, Sivakasi- 626124
Tamil Nadu, India.
Subscribe to my YouTube channel: https://www.youtube.com/c/OneChemistry?sub_confirmation=1
Content Creation: Prof. GKR
Please feel free to give comments and feedback.
__________________________________________________________________________
The creator of this channel Prof. GKR, his Ph.D in Chemistry from Hyderabad Central University and cleared CSIR-JRF and NET. He is currently Associate Professor in the P.S.R Arts and Science College, Sivakasi, Tamil Nadu.
## Know One Chemistry ##
Youtube : https://www.youtube.com/c/OneChemistry
Facebook: https://www.facebook.com/wingsGKR/
Instagram: https://www.instagram.com/onechemistrygkr/
Twitter: https://twitter.com/OneChemistry
Website: https://sites.google.com/view/suviganu/home
Email: suviganu@gmail.com
___________________________________________________________________________
#chemistry #chemistryconcepts #jeeneetchemistry #organicchemistry #TRB #gate #netexam #chemistrynotes #chemicalreactions #organicchemistrytricks #neet #jee #setexam #setexampreparation #netexampreparation #gateexam #gateexampreparation #netexampreparation #neetexam #neetchemistry #neetchemistrypreparation #gatechemistry #netchemistry #ugcchemistry #jee #jeechemistry #jeechemistryquestions #jamchemistry
The content may help you to clear
UPSC Chemistry
CSIR Chemical Science
UGC Chemical Science
GATE Chemistry
NET Chemical Science
NEET Chemistry
JAM Chemistry
TNPSC Chemistry
TRB CHEMISTRY
JEE Chemistry
This video is about annelation effect in aromatic system. The annealtion effect is partial loss of aromatic character due to the sharing of electron in polycyclic system.
The annelation effect in chemistry refers to the stabilization or destabilization that occurs when a ring is fused to another molecule. This effect is primarily observed in aromatic compounds and is influenced by factors such as ring size, aromaticity, and the nature of the fused rings.
Aromaticity: The annelation effect is particularly pronounced in aromatic compounds. Fusing a ring to an aromatic system can either enhance or diminish its aromaticity.
Ring Size: The size of the fused ring plays a crucial role. Fusing a five-membered or six-membered ring to an aromatic system generally leads to stabilization, while fusing larger or smaller rings can result in destabilization.
Nature of Fused Rings: The electronic properties of the fused rings also influence the annelation effect. Electron-rich fused rings tend to stabilize the aromatic system, while electron-deficient rings can destabilize it.
Cases of annelation effect:
Naphthalene: When a benzene ring is fused to another benzene ring to form naphthalene, the resulting molecule is more stable than two isolated benzene rings. This is due to the delocalization of electrons over the entire fused system.
Anthracene: Fusing another benzene ring to naphthalene results in anthracene, which is also more stable than its constituent benzene rings. However, the stabilization is not as significant as in naphthalene due to the increased steric hindrance between the fused rings.
Phenanthrene: Fusing a benzene ring to a different position of naphthalene leads to phenanthrene, which is less stable than naphthalene. This is because the fusion introduces some strain into the molecule.
Factors influencing the annelation effect:
Resonance stabilization: The delocalization of electrons through resonance contributes to the stability of fused aromatic systems.
Angle strain: The angles between bonds in fused rings can deviate from the ideal 120° angle, leading to strain and destabilization.
Steric hindrance: Bulky substituents on the fused rings can hinder rotation and increase steric repulsion, destabilizing the molecule.
This video is part of one minute chemistry series. Enjoy Quick learning.
For business queries, send message to suviganu@gmail.com with your phone number . We will call you back.
Office Address
Dr. B. Ganesh Kumar,
1255, Third street, Asari Colony,
Satchiyapuram, Sivakasi- 626124
Tamil Nadu, India.
Subscribe to my YouTube channel: https://www.youtube.com/c/OneChemistry?sub_confirmation=1
Content Creation: Prof. GKR
Please feel free to give comments and feedback.
__________________________________________________________________________
The creator of this channel Prof. GKR, his Ph.D in Chemistry from Hyderabad Central University and cleared CSIR-JRF and NET. He is currently Associate Professor in the P.S.R Arts and Science College, Sivakasi, Tamil Nadu.
## Know One Chemistry ##
Youtube : https://www.youtube.com/c/OneChemistry
Facebook: https://www.facebook.com/wingsGKR/
Instagram: https://www.instagram.com/onechemistrygkr/
Twitter: https://twitter.com/OneChemistry
Website: https://sites.google.com/view/suviganu/home
Email: suviganu@gmail.com
___________________________________________________________________________
#chemistry #chemistryconcepts #jeeneetchemistry #organicchemistry #TRB #gate #netexam #chemistrynotes #chemicalreactions #organicchemistrytricks #neet #jee #setexam #setexampreparation #netexampreparation #gateexam #gateexampreparation #netexampreparation #neetexam #neetchemistry #neetchemistrypreparation #gatechemistry #netchemistry #ugcchemistry #jee #jeechemistry #jeechemistryquestions #jamchemistry
The content may help you to clear
UPSC Chemistry
CSIR Chemical Science
UGC Chemical Science
GATE Chemistry
NET Chemical Science
NEET Chemistry
JAM Chemistry
TNPSC Chemistry
TRB CHEMISTRY
JEE Chemistry
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
3.3K
Likes
101
Duration
1:37
Published
Feb 12, 2022
User Reviews
4.6
(3) Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.