Sharpless Asymmetric Epoxidation: Mechanism, Applications & Key Concepts 🧪
Learn about the Sharpless epoxidation process, its mechanism, real-world applications, and how it plays a vital role in asymmetric synthesis. Perfect for GATE, NET, and SET exam preparation!

One Chemistry
268 views • Apr 6, 2025
About this video
For feedback and business queries, please email us at suviganu@gmail.com
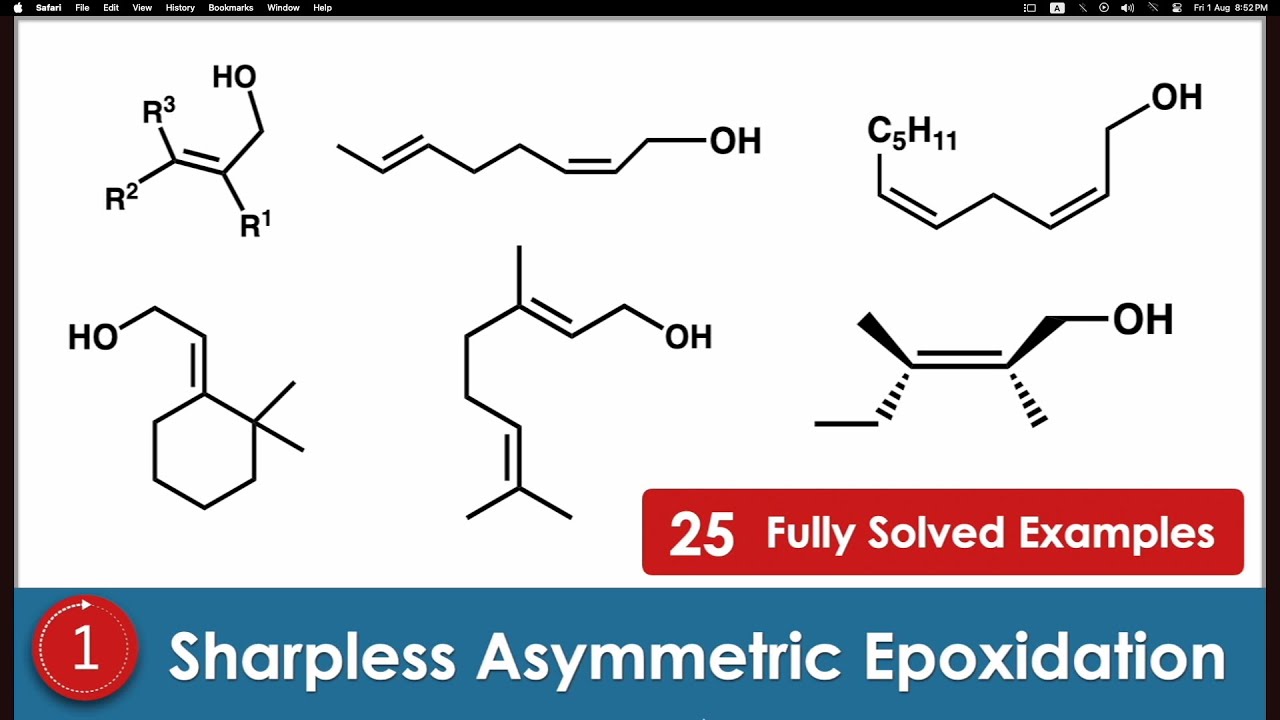
Sharpless epoxidation is an enantioselective chemical reaction that converts primary and secondary allylic alcohols into chiral epoxides using titanium tetraisopropoxide (Ti(OiPr)₄), an enantiopure tartrate ligand (such as diethyl tartrate), and an oxidant like tert-butyl hydroperoxide. Developed by K. Barry Sharpless, this reaction is widely used in asymmetric synthesis to produce optically pure epoxides, which serve as valuable intermediates in the pharmaceutical and fine chemical industries. The choice of (R,R)- or (S,S)-diethyl tartrate controls the enantiomeric outcome, making it a highly effective method for stereoselective epoxidation.
Reaction Overview
Allylic Alcohol + t-BuOOH + Ti(OiPr) 4 + Tartrate → Chiral Epoxide
Key Reagents:
Titanium Tetraisopropoxide (Ti(OiPr)₄): Acts as the Lewis acid catalyst, coordinating with the tartrate ligand and the allylic alcohol.
Enantiopure Tartrate Ligand:
(R,R)-Diethyl tartrate (DET) or (S,S)-Diethyl tartrate (DET) controls the stereochemistry of the epoxide.
(R,R)-DET leads to one enantiomer, while (S,S)-DET gives the opposite enantiomer.
Tert-Butyl Hydroperoxide (t-BuOOH): Serves as the oxygen source for epoxidation.
Mechanism of the Sharpless Epoxidation
Formation of the Catalytic Complex:
The titanium(IV) complex forms by coordinating Ti(OiPr)₄ with the tartrate ligand.
The allylic alcohol also coordinates with titanium, activating it for epoxidation.
Transfer of the Oxygen Atom:
The hydroperoxide group from t-BuOOH interacts with the titanium center.
This leads to a concerted oxygen transfer to the double bond of the allylic alcohol.
The reaction follows a stereoelectronic model, where the tartrate ligand directs the approach of the oxidant to the double bond, ensuring high enantioselectivity.
Formation of the Chiral Epoxide:
The epoxide product is released, and the catalytic cycle continues with another substrate molecule.
Stereochemistry Control
The absolute configuration of the epoxide is controlled by the choice of tartrate ligand:
R,R-Diethyl tartrate (DET) → Gives one enantiomer.
(S,S)-Diethyl tartrate (DET) → Gives the opposite enantiomer.
The Sharpless mnemonic helps predict the enantioselectivity:
When viewed with the OH group of the allylic alcohol at the top, the epoxide forms above or below the plane based on the DET used.
Applications of Sharpless Epoxidation
Pharmaceutical Synthesis: Many chiral drugs require enantiopure epoxides as intermediates, such as β-blockers and antifungal agents.
Natural Product Synthesis: Used to prepare epoxide intermediates in complex natural molecules like prostaglandins and macrolides.
Fine Chemicals and Agrochemicals: Chiral epoxides are crucial for making herbicides, pesticides, and other functional molecules.
Advantages of Sharpless Epoxidation
Highly Enantioselective: Produces almost exclusively one enantiomer (90% ee).
Mild Reaction Conditions: Works at room temperature in non-aqueous solvents.
Broad Substrate Scope: Works for a wide range of allylic alcohols.
Limitations
Only works efficiently with allylic alcohols; simple alkenes do not undergo selective epoxidation.
Uses stoichiometric amounts of Ti(OiPr)₄, which can be expensive.
The video may include the following topics
Introduction to Sharpless Asymmetric Epoxidation
Sharpless Epoxidation: A Landmark in Asymmetric Synthesis
Mechanism of Sharpless Asymmetric Epoxidation Explained
The Role of Titanium and Tartrate in Sharpless Epoxidation
Enantioselectivity in Sharpless Asymmetric Epoxidation
Applications of Sharpless Epoxidation in Organic Synthesis
Sharpless Epoxidation: Reagents, Conditions, and Stereochemistry
Why Sharpless Epoxidation is Important in Modern Chemistry
Sharpless Epoxidation: A Tool for Making Chiral Epoxides
Understanding the Stereochemical Outcome in Sharpless Epoxidation
Sharpless Epoxidation
Asymmetric Synthesis
Chiral Epoxides
Enantioselective Reaction
Organic Chemistry
K Barry Sharpless
Epoxidation Reaction
Titanium Catalysis
Diethyl Tartrate
Stereoselective Synthesis
tBuOOH
Chiral Catalysis
Allylic Alcohols
The video is Ideal for
University Chemistry Exams
Competitive Exams Like
GATE Chemistry
NET Chemical Science
UGC Chemical Science
SET Chemistry
JEE Chemistry
JAM Chemistry
NEET Chemistry
#OrganicChemistry
#ChemistryExplained
#ChemicalReactions
#LearnChemistry
#MolecularScience
#ChemistryTutorials
#ScienceExperiments
#InorganicChemistry
#PhysicalChemistry
#ChemistryFacts
#OrganicReactions
#ReactionMechanism
#ChemistryMechanisms
#AdvancedOrganicChemistry
#ChemistryExamPrep
#CompetitiveChemistry
#ChemistryMCQs
#JEEChemistry
#NEETChemistry
#ChemistryRevision
#PhysicalChemistryTricks
#OrganicChemistryShortcuts
#ChemistryStudyTips
#competitiveexampreparation
Sharpless epoxidation is an enantioselective chemical reaction that converts primary and secondary allylic alcohols into chiral epoxides using titanium tetraisopropoxide (Ti(OiPr)₄), an enantiopure tartrate ligand (such as diethyl tartrate), and an oxidant like tert-butyl hydroperoxide. Developed by K. Barry Sharpless, this reaction is widely used in asymmetric synthesis to produce optically pure epoxides, which serve as valuable intermediates in the pharmaceutical and fine chemical industries. The choice of (R,R)- or (S,S)-diethyl tartrate controls the enantiomeric outcome, making it a highly effective method for stereoselective epoxidation.
Reaction Overview
Allylic Alcohol + t-BuOOH + Ti(OiPr) 4 + Tartrate → Chiral Epoxide
Key Reagents:
Titanium Tetraisopropoxide (Ti(OiPr)₄): Acts as the Lewis acid catalyst, coordinating with the tartrate ligand and the allylic alcohol.
Enantiopure Tartrate Ligand:
(R,R)-Diethyl tartrate (DET) or (S,S)-Diethyl tartrate (DET) controls the stereochemistry of the epoxide.
(R,R)-DET leads to one enantiomer, while (S,S)-DET gives the opposite enantiomer.
Tert-Butyl Hydroperoxide (t-BuOOH): Serves as the oxygen source for epoxidation.
Mechanism of the Sharpless Epoxidation
Formation of the Catalytic Complex:
The titanium(IV) complex forms by coordinating Ti(OiPr)₄ with the tartrate ligand.
The allylic alcohol also coordinates with titanium, activating it for epoxidation.
Transfer of the Oxygen Atom:
The hydroperoxide group from t-BuOOH interacts with the titanium center.
This leads to a concerted oxygen transfer to the double bond of the allylic alcohol.
The reaction follows a stereoelectronic model, where the tartrate ligand directs the approach of the oxidant to the double bond, ensuring high enantioselectivity.
Formation of the Chiral Epoxide:
The epoxide product is released, and the catalytic cycle continues with another substrate molecule.
Stereochemistry Control
The absolute configuration of the epoxide is controlled by the choice of tartrate ligand:
R,R-Diethyl tartrate (DET) → Gives one enantiomer.
(S,S)-Diethyl tartrate (DET) → Gives the opposite enantiomer.
The Sharpless mnemonic helps predict the enantioselectivity:
When viewed with the OH group of the allylic alcohol at the top, the epoxide forms above or below the plane based on the DET used.
Applications of Sharpless Epoxidation
Pharmaceutical Synthesis: Many chiral drugs require enantiopure epoxides as intermediates, such as β-blockers and antifungal agents.
Natural Product Synthesis: Used to prepare epoxide intermediates in complex natural molecules like prostaglandins and macrolides.
Fine Chemicals and Agrochemicals: Chiral epoxides are crucial for making herbicides, pesticides, and other functional molecules.
Advantages of Sharpless Epoxidation
Highly Enantioselective: Produces almost exclusively one enantiomer (90% ee).
Mild Reaction Conditions: Works at room temperature in non-aqueous solvents.
Broad Substrate Scope: Works for a wide range of allylic alcohols.
Limitations
Only works efficiently with allylic alcohols; simple alkenes do not undergo selective epoxidation.
Uses stoichiometric amounts of Ti(OiPr)₄, which can be expensive.
The video may include the following topics
Introduction to Sharpless Asymmetric Epoxidation
Sharpless Epoxidation: A Landmark in Asymmetric Synthesis
Mechanism of Sharpless Asymmetric Epoxidation Explained
The Role of Titanium and Tartrate in Sharpless Epoxidation
Enantioselectivity in Sharpless Asymmetric Epoxidation
Applications of Sharpless Epoxidation in Organic Synthesis
Sharpless Epoxidation: Reagents, Conditions, and Stereochemistry
Why Sharpless Epoxidation is Important in Modern Chemistry
Sharpless Epoxidation: A Tool for Making Chiral Epoxides
Understanding the Stereochemical Outcome in Sharpless Epoxidation
Sharpless Epoxidation
Asymmetric Synthesis
Chiral Epoxides
Enantioselective Reaction
Organic Chemistry
K Barry Sharpless
Epoxidation Reaction
Titanium Catalysis
Diethyl Tartrate
Stereoselective Synthesis
tBuOOH
Chiral Catalysis
Allylic Alcohols
The video is Ideal for
University Chemistry Exams
Competitive Exams Like
GATE Chemistry
NET Chemical Science
UGC Chemical Science
SET Chemistry
JEE Chemistry
JAM Chemistry
NEET Chemistry
#OrganicChemistry
#ChemistryExplained
#ChemicalReactions
#LearnChemistry
#MolecularScience
#ChemistryTutorials
#ScienceExperiments
#InorganicChemistry
#PhysicalChemistry
#ChemistryFacts
#OrganicReactions
#ReactionMechanism
#ChemistryMechanisms
#AdvancedOrganicChemistry
#ChemistryExamPrep
#CompetitiveChemistry
#ChemistryMCQs
#JEEChemistry
#NEETChemistry
#ChemistryRevision
#PhysicalChemistryTricks
#OrganicChemistryShortcuts
#ChemistryStudyTips
#competitiveexampreparation
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
268
Likes
8
Duration
43:48
Published
Apr 6, 2025
Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.