Understanding Magnetic & Spin Quantum Numbers: Key to Electron Behavior 🔍
Learn about the four orbital quantum numbers, including magnetic and spin quantum numbers, and how they define electron behavior in atoms. Perfect for students and enthusiasts!
Punjab Group Of Colleges
77 views • Jan 12, 2016
About this video
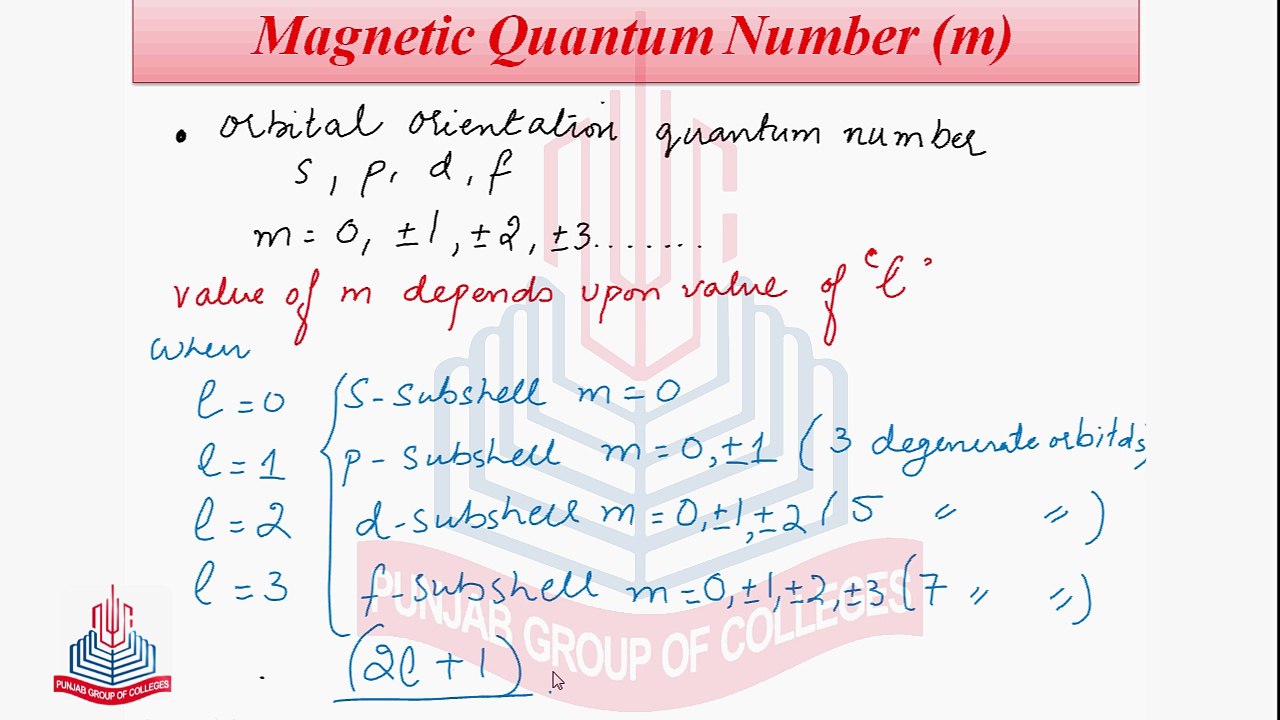
Concept of Orbital <br />Quantum Numbers : <br />A set of integral numbers which describes the behavior of an electron in an orbital . <br />There are four types <br />1. Principal Quantum Number (n) <br />2. Azimuthal Quantum Number (l) <br />3. Magnetic Quantum Number (m) <br /> <br />4. Spin Quantum Number (s) <br />Chapter No 5 <br />Atomic Structure <br />chemistry <br />Part 1
Video Information
Views
77
Duration
13:59
Published
Jan 12, 2016
Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.
Trending Now