Understanding Crystal Symmetry in Solid State Physics 🧊
Learn the fundamental principles of crystal symmetry in solid state physics. Download our Android app for more videos and stay updated on physical chemistry topics!

Edmerls
136.5K views • Apr 17, 2018
About this video
Download our Android app at https://goo.gl/5JM1G2
To Get New Videos on WhatsApp please fill the form at
https://goo.gl/forms/w5jpVKuyjT2JdTIY2
“Every crystal shows various types of symmetry.”
“All crystals of the same substance possess the same elements of symmetry.”
When a crystal is rotated about its axis, it shows the same appearance it is known as crystal symmetry.
Various types of symmetry in a crystal is known as elements of symmetry.
Centre of Symmetry:It is defined as an imaginary point within the crystal such that any line passing through this point intersects the opposite face of the crystal at equal distances.
In other words, any line drawn through this point will intersect surface at equal distance in both the directions.
Plane of Symmetry:It is an imaginary plane which divides the crystal into two equal parts such that one is the mirror image of the other.
For example, regular cubic structure can have 9 planes of symmetry. Three planes of symmetry are parallel to the cube faces and six diagonal planes are passing through opposite cube edges.
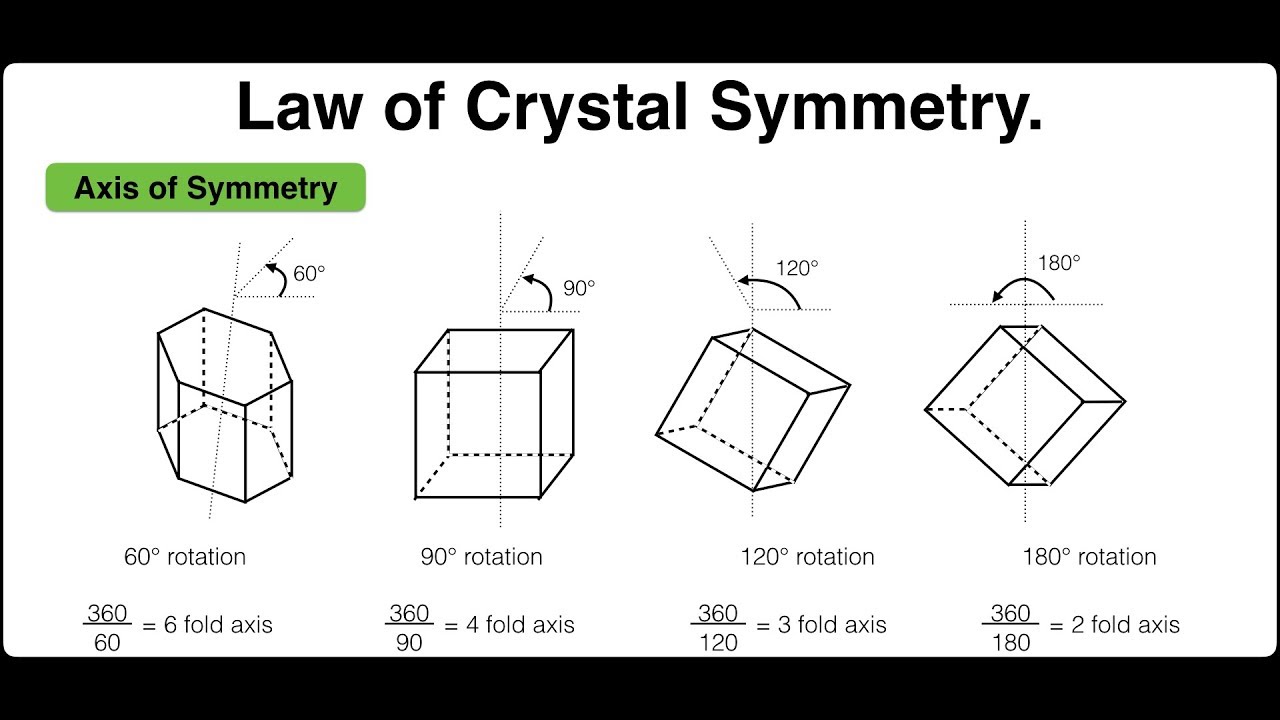
Axis of Symmetry:Axis of symmetry is a line about which the crystal may be rotated such that it presents the same appearance more than once during the complete revolution through 360o.
If a crystal presents the same appearance ’n’ times in one complete revolution, the axis is said to be ’n’ fold symmetry.
E.g. the cubic crystal of NaCl has 13 axes of symmetry out of which 4 are threefold, 3 are fourfold and 6 are twofold.
For Details Visit
http://cepekmedia.co.nf
http://cepek.hol.es/
http://edmerls.66Ghz.com/
http://edmerls.tk/
To Get New Videos on WhatsApp please fill the form at
https://goo.gl/forms/w5jpVKuyjT2JdTIY2
“Every crystal shows various types of symmetry.”
“All crystals of the same substance possess the same elements of symmetry.”
When a crystal is rotated about its axis, it shows the same appearance it is known as crystal symmetry.
Various types of symmetry in a crystal is known as elements of symmetry.
Centre of Symmetry:It is defined as an imaginary point within the crystal such that any line passing through this point intersects the opposite face of the crystal at equal distances.
In other words, any line drawn through this point will intersect surface at equal distance in both the directions.
Plane of Symmetry:It is an imaginary plane which divides the crystal into two equal parts such that one is the mirror image of the other.
For example, regular cubic structure can have 9 planes of symmetry. Three planes of symmetry are parallel to the cube faces and six diagonal planes are passing through opposite cube edges.
Axis of Symmetry:Axis of symmetry is a line about which the crystal may be rotated such that it presents the same appearance more than once during the complete revolution through 360o.
If a crystal presents the same appearance ’n’ times in one complete revolution, the axis is said to be ’n’ fold symmetry.
E.g. the cubic crystal of NaCl has 13 axes of symmetry out of which 4 are threefold, 3 are fourfold and 6 are twofold.
For Details Visit
http://cepekmedia.co.nf
http://cepek.hol.es/
http://edmerls.66Ghz.com/
http://edmerls.tk/
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
136.5K
Likes
2.8K
Duration
3:30
Published
Apr 17, 2018
User Reviews
4.7
(27) Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.