Master Metal-Metal Bond Counting with the 18 Electron Rule in 1 Minute 🧪
Learn how to quickly calculate metal-metal bonds using the 18 Electron Rule and bond order formulas. Perfect for chemistry students and enthusiasts seeking a clear, concise explanation!

One Chemistry
4.3K views • Apr 22, 2022
About this video
For feedback and business queries, please email us at suviganu@gmail.com
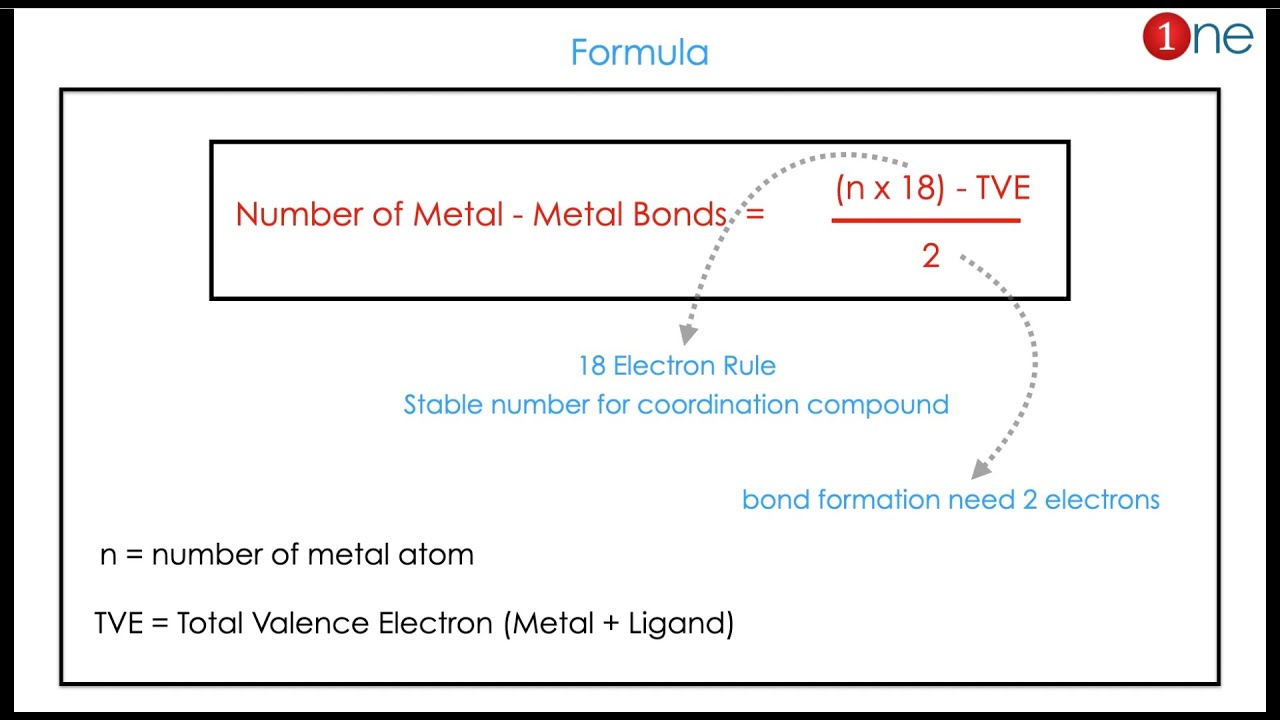
This video help you to calculate the total number of metal metal bonds (M-M). They are also called as metal cluster. Counting of metal metal bond in cluster is important parameter to predict the structure in complex compounds or coordination compounds. The formula derived based on the skeletal electron with stable 18 electron concept. The 18 electron rule is base for the prediction of the metal metal bond.
Coordination compounds often involve multiple metal centers connected by metal-metal bonds. Accurately counting these bonds is crucial for understanding the structure and reactivity of these complexes. Here are some key concepts and methods for counting metal-metal bonds in coordination chemistry:
1. Bond Order:
Metal-metal bonds can have various bond orders, from single to multiple bonds.
Single bonds are generally formed by the overlap of two d orbitals, while multiple bonds involve additional interactions such as π-bonding.
Bond order can be inferred from experimental data like bond lengths and spectroscopic properties.
2. Structural Considerations:
The geometry of the metal cluster plays a significant role in determining the number and type of metal-metal bonds.
Close metal-metal distances and shared bridging ligands often indicate the presence of metal-metal bonds.
Structural analysis techniques like X-ray crystallography are indispensable for visualizing metal-metal bonds.
3. Electron Counting Rules:
Several electron counting rules have been developed to predict the number of metal-metal bonds in coordination compounds.
These rules often involve counting the total number of valence electrons contributed by the metal atoms and ligands.
Common rules include the 18-electron rule and the effective atomic number (EAN) rule.
4. Spectroscopic Analysis:
Spectroscopic techniques like IR, Raman, and NMR spectroscopy can provide valuable information about the presence and nature of metal-metal bonds.
For example, vibrational spectroscopy can reveal the presence of metal-metal stretching vibrations.
5. Computational Methods:
Density functional theory (DFT) and other computational methods can be used to calculate bond orders and predict the presence of metal-metal bonds.
These methods can provide insights into the electronic structure and bonding interactions in coordination compounds.
This video is part of one minute chemistry series. Enjoy Quick learning.
For business queries, send message to suviganu@gmail.com with your phone number. We will call you back.
Office Address
Dr. B. Ganesh Kumar,
1255, Third street, Asari Colony,
Satchiyapuram, Sivakasi- 626124
Tamil Nadu, India.
#JEE #NEET #NET #GATE #One #OneChemistry #JAM #Chemistry
Subscribe to my YouTube channel: https://www.youtube.com/c/OneChemistry?sub_confirmation=1
Content Creation: Prof. GKR
Please feel free to give comments and feedback.
__________________________________________________________________________
The creator of this channel Prof. GKR, his Ph.D in Chemistry from Hyderabad Central University and cleared CSIR-JRF and NET. He is currently Associate Professor in the P.S.R Arts and Science College, Sivakasi, Tamil Nadu.
## Know One Chemistry ##
Youtube : https://www.youtube.com/c/OneChemistry
Facebook: https://www.facebook.com/wingsGKR/
Instagram: https://www.instagram.com/onechemistrygkr/
Twitter: https://twitter.com/OneChemistry
Website: https://sites.google.com/view/suviganu/home
Email: suviganu@gmail.com
___________________________________________________________________________
The content may include basic and advanced organic chemistry questions. Which may appear in the following Examinations
UPSC Chemistry previous Year questions
CSIR Chemical Science previous Year questions
UGC Chemical Science previous Year questions
GATE Chemistry previous Year questions
NET Chemical Science previous Year questions
JAM Chemistry previous Year questions
#chemistry #chemistryconcepts #jeeneetchemistry #organicchemistry #TRB #gate #netexam #chemistrynotes #chemicalreactions #organicchemistrytricks #neet #jee #setexam #setexampreparation #netexampreparation #gateexam #gateexampreparation #netexampreparation #neetexam #neetchemistry #neetchemistrypreparation #gatechemistry #netchemistry #ugcchemistry #jee #jeechemistry #jeechemistryquestions #jamchemistry
The content may help you to clear
UPSC Chemistry
CSIR Chemical Science
UGC Chemical Science
GATE Chemistry
NET Chemical Science
NEET Chemistry
JAM Chemistry
TNPSC Chemistry
TRB CHEMISTRY
JEE Chemistry
This video help you to calculate the total number of metal metal bonds (M-M). They are also called as metal cluster. Counting of metal metal bond in cluster is important parameter to predict the structure in complex compounds or coordination compounds. The formula derived based on the skeletal electron with stable 18 electron concept. The 18 electron rule is base for the prediction of the metal metal bond.
Coordination compounds often involve multiple metal centers connected by metal-metal bonds. Accurately counting these bonds is crucial for understanding the structure and reactivity of these complexes. Here are some key concepts and methods for counting metal-metal bonds in coordination chemistry:
1. Bond Order:
Metal-metal bonds can have various bond orders, from single to multiple bonds.
Single bonds are generally formed by the overlap of two d orbitals, while multiple bonds involve additional interactions such as π-bonding.
Bond order can be inferred from experimental data like bond lengths and spectroscopic properties.
2. Structural Considerations:
The geometry of the metal cluster plays a significant role in determining the number and type of metal-metal bonds.
Close metal-metal distances and shared bridging ligands often indicate the presence of metal-metal bonds.
Structural analysis techniques like X-ray crystallography are indispensable for visualizing metal-metal bonds.
3. Electron Counting Rules:
Several electron counting rules have been developed to predict the number of metal-metal bonds in coordination compounds.
These rules often involve counting the total number of valence electrons contributed by the metal atoms and ligands.
Common rules include the 18-electron rule and the effective atomic number (EAN) rule.
4. Spectroscopic Analysis:
Spectroscopic techniques like IR, Raman, and NMR spectroscopy can provide valuable information about the presence and nature of metal-metal bonds.
For example, vibrational spectroscopy can reveal the presence of metal-metal stretching vibrations.
5. Computational Methods:
Density functional theory (DFT) and other computational methods can be used to calculate bond orders and predict the presence of metal-metal bonds.
These methods can provide insights into the electronic structure and bonding interactions in coordination compounds.
This video is part of one minute chemistry series. Enjoy Quick learning.
For business queries, send message to suviganu@gmail.com with your phone number. We will call you back.
Office Address
Dr. B. Ganesh Kumar,
1255, Third street, Asari Colony,
Satchiyapuram, Sivakasi- 626124
Tamil Nadu, India.
#JEE #NEET #NET #GATE #One #OneChemistry #JAM #Chemistry
Subscribe to my YouTube channel: https://www.youtube.com/c/OneChemistry?sub_confirmation=1
Content Creation: Prof. GKR
Please feel free to give comments and feedback.
__________________________________________________________________________
The creator of this channel Prof. GKR, his Ph.D in Chemistry from Hyderabad Central University and cleared CSIR-JRF and NET. He is currently Associate Professor in the P.S.R Arts and Science College, Sivakasi, Tamil Nadu.
## Know One Chemistry ##
Youtube : https://www.youtube.com/c/OneChemistry
Facebook: https://www.facebook.com/wingsGKR/
Instagram: https://www.instagram.com/onechemistrygkr/
Twitter: https://twitter.com/OneChemistry
Website: https://sites.google.com/view/suviganu/home
Email: suviganu@gmail.com
___________________________________________________________________________
The content may include basic and advanced organic chemistry questions. Which may appear in the following Examinations
UPSC Chemistry previous Year questions
CSIR Chemical Science previous Year questions
UGC Chemical Science previous Year questions
GATE Chemistry previous Year questions
NET Chemical Science previous Year questions
JAM Chemistry previous Year questions
#chemistry #chemistryconcepts #jeeneetchemistry #organicchemistry #TRB #gate #netexam #chemistrynotes #chemicalreactions #organicchemistrytricks #neet #jee #setexam #setexampreparation #netexampreparation #gateexam #gateexampreparation #netexampreparation #neetexam #neetchemistry #neetchemistrypreparation #gatechemistry #netchemistry #ugcchemistry #jee #jeechemistry #jeechemistryquestions #jamchemistry
The content may help you to clear
UPSC Chemistry
CSIR Chemical Science
UGC Chemical Science
GATE Chemistry
NET Chemical Science
NEET Chemistry
JAM Chemistry
TNPSC Chemistry
TRB CHEMISTRY
JEE Chemistry
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
4.3K
Likes
30
Duration
1:46
Published
Apr 22, 2022
User Reviews
4.1
(4) Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.
Trending Now