How to Calculate pH When Mixing Two Buffer Solutions 🧪
Learn step-by-step how to determine the pH of a buffer solution created by combining sodium dihydrogen citrate and sodium hydrogen citrate, using their concentrations and pKa value.

University Aid
12.4K views • Dec 4, 2014
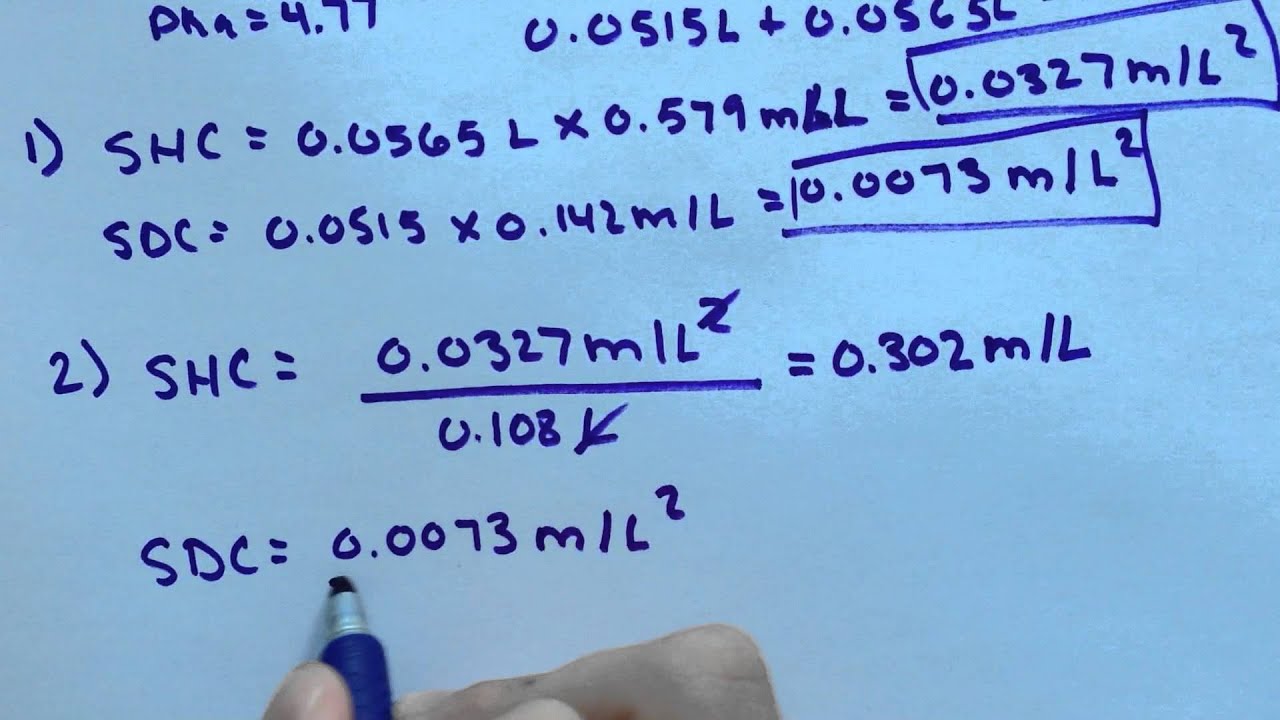
About this video
The question
-A buffer solution is prepared by mixing 51.5mL of 0.142M sodium dihydrogen citrate with 56.5mL of 0.579M sodium hydrogen citrate. Your Pka = 4.77. Calculate the pH.
-A buffer solution is prepared by mixing 51.5mL of 0.142M sodium dihydrogen citrate with 56.5mL of 0.579M sodium hydrogen citrate. Your Pka = 4.77. Calculate the pH.
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
12.4K
Likes
27
Duration
7:38
Published
Dec 4, 2014
User Reviews
3.9
(2) Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.
Trending Now