FDA SUSAR Reporting Essentials for Sponsors
Learn the key steps for sponsors to report Serious and Unexpected Suspected Adverse Reactions (SUSARs) to the FDA.

Regulatory Affairs 101
12 views • Nov 15, 2025
About this video
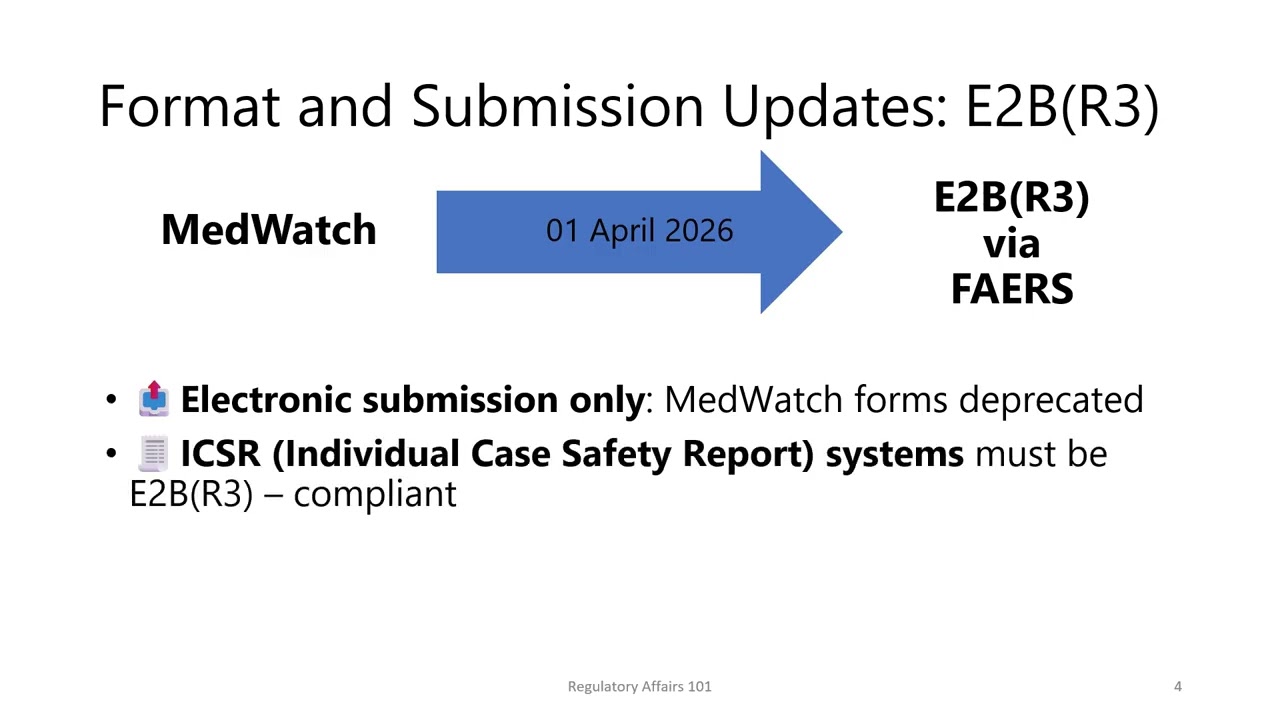
In this video, we are exploring the essentials of FDA reporting for Serious and Unexpected Suspected Adverse Reactions, or SUSARs - specifically for sponsors conducting clinical trials under an Investigational New Drug application, or IND. We will cover what qualifies as a SUSAR, why timely and accurate reporting is critical, and how to ensure compliance throughout your clinical trial lifecycle.
Video Information
Views
12
Likes
1
Duration
5:26
Published
Nov 15, 2025
Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.
Trending Now