FDA Human Factors Guidance Made Easy 📋
Learn a simple, step-by-step method to understand the FDA Human Factors Guidance, covering key components for compliance.

Research Collective
1.8K views • Jul 18, 2023
About this video
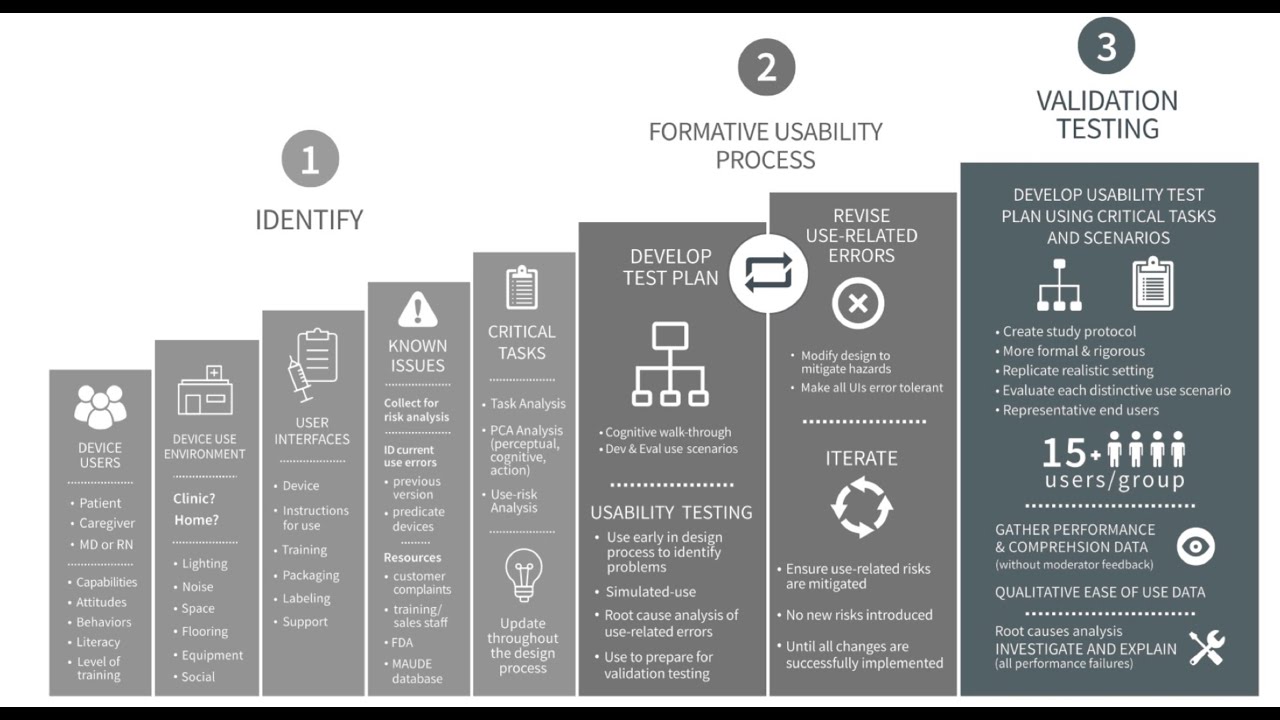
This video provides a simple, step-by-step approach to the FDA Human Factors Guidance. It discusses the three main components of the approach: 1) - Identifying and characterizing users, uses, use environments, user interfaces, known issues, and critical tasks, 2) Formative usability testing, and 3) Validation usability testing. It also discusses the components of the Human Factors and Usability Engineering report and provides advice on how to keep that simple.
This video, provided by @researchcollective, a healthcare and medical device consulting firm, is useful for healthcare and medical device Human Factors Engineers, User Experience (UX) designers and researchers, and Quality Engineers.
Chapters
00:01 Introduction to FDA Human Factors Guidance
00:48 History of the FDA Human Factors Guidance
02:10 Three Phases of the FDA Guidance
02:35 Identifying and Characterizing Users, Environments, Interfaces, Known Issues and Critical Tasks
10:04 Formative Usability Testing
12:41 Validation Usability Testing
14:15 Data Analysis and Reporting
15:58 Post-market Surveillance
16:35 Summary
This video, provided by @researchcollective, a healthcare and medical device consulting firm, is useful for healthcare and medical device Human Factors Engineers, User Experience (UX) designers and researchers, and Quality Engineers.
Chapters
00:01 Introduction to FDA Human Factors Guidance
00:48 History of the FDA Human Factors Guidance
02:10 Three Phases of the FDA Guidance
02:35 Identifying and Characterizing Users, Environments, Interfaces, Known Issues and Critical Tasks
10:04 Formative Usability Testing
12:41 Validation Usability Testing
14:15 Data Analysis and Reporting
15:58 Post-market Surveillance
16:35 Summary
Tags and Topics
Browse our collection to discover more content in these categories.
Video Information
Views
1.8K
Likes
47
Duration
18:29
Published
Jul 18, 2023
User Reviews
4.5
(1) Related Trending Topics
LIVE TRENDSRelated trending topics. Click any trend to explore more videos.